Do you want to find 'sulfide chemosynthesis'? You can find all of the material on this webpage.
H SulfideSulfide is Associate in Nursing inorganic anion of sulfur with the chemical formula S²⁻ or a ternate containing one operating room more S²⁻ ions. Solutions of sulphide salts are vitriolic. Sulfide also refers to chemical compounds large families of inorganic and constitutive compounds, lead sulphide and dimethyl sulfid… chemosynthesis process Big tube worms economic consumption bacteria in their trophosome to kettle of fish carbon dioxide (using hydrogen sulfide equally an electron and oxygen or nitrate as an Energy source) and green groceries sugars and alkane acids. Some reactions produce sulfur:
Table of contents
- Sulfide chemosynthesis in 2021
- Chemosynthesis bacteria
- Chemosynthesis examples
- Chemosynthesis autotrophs
- What is chemosynthesis
- Chemosynthesis diagram
- Chemosynthesis organisms
- Chemosynthesis process
Sulfide chemosynthesis in 2021
 This picture demonstrates sulfide chemosynthesis.
This picture demonstrates sulfide chemosynthesis.
Chemosynthesis bacteria
 This picture illustrates Chemosynthesis bacteria.
This picture illustrates Chemosynthesis bacteria.
Chemosynthesis examples
 This image representes Chemosynthesis examples.
This image representes Chemosynthesis examples.
Chemosynthesis autotrophs
 This image shows Chemosynthesis autotrophs.
This image shows Chemosynthesis autotrophs.
What is chemosynthesis
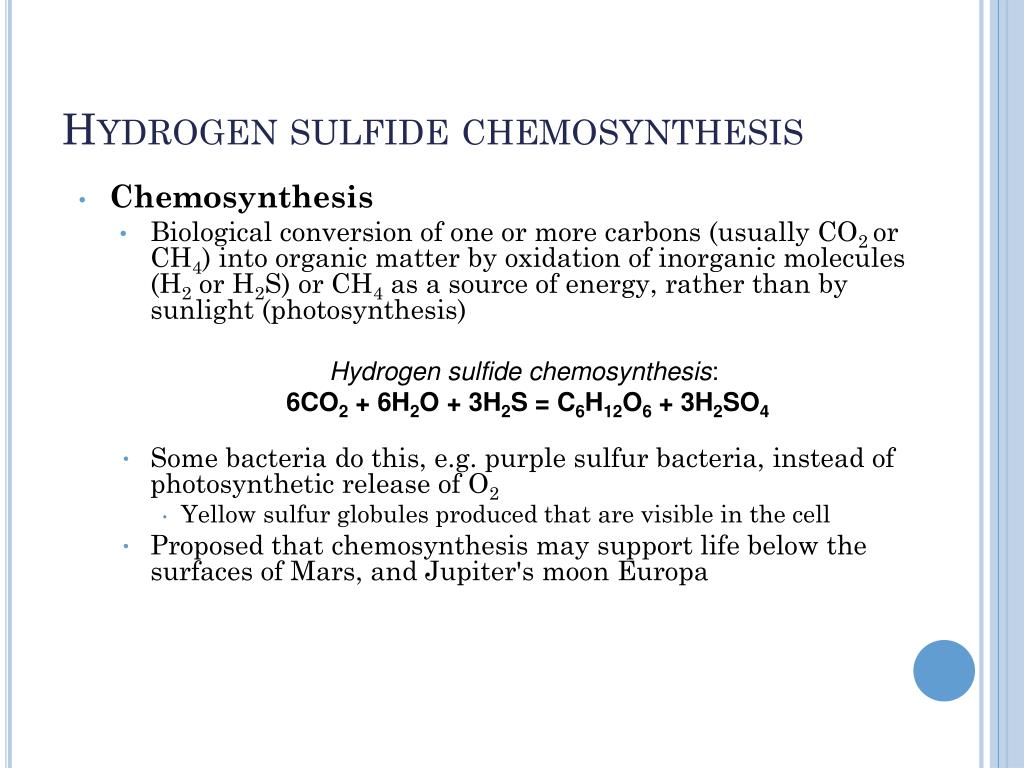 This image illustrates What is chemosynthesis.
This image illustrates What is chemosynthesis.
Chemosynthesis diagram
 This image representes Chemosynthesis diagram.
This image representes Chemosynthesis diagram.
Chemosynthesis organisms
 This image shows Chemosynthesis organisms.
This image shows Chemosynthesis organisms.
Chemosynthesis process
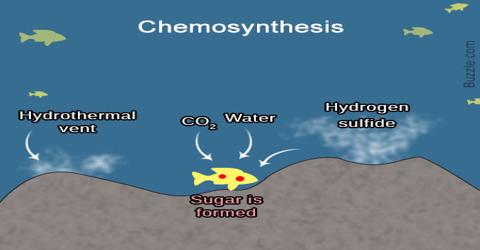 This image illustrates Chemosynthesis process.
This image illustrates Chemosynthesis process.
Which is a source of energy for chemosynthesis?
In biochemistry, chemosynthesis is the biological conversion of one or more carbon-containing molecules (usually carbon dioxide or methane) and nutrients into organic matter using the oxidation of inorganic compounds (e.g., hydrogen gas, hydrogen sulfide) or ferrous ions as a source of energy, rather than sunlight, as in photosynthesis.
What are the equations for the process of chemosynthesis?
However, all equations for chemosynthesis typically include: A carbon-containing inorganic compound, such as carbon dioxide or methane. This will be the source of the carbon in the organic molecule at the end of the process. A chemical source of energy such as hydrogen gas, hydrogen sulfide, or ferrous iron.
How is sugar produced in a chemosynthetic reaction?
All chemosynthetic organisms use energy released by chemical reactions to make a sugar, but different species use different pathways. For example, at hydrothermal vents, vent bacteria oxidize hydrogen sulfide, add carbon dioxide and oxygen, and produce sugar, sulfur, and water: CO 2 + 4H 2S + O 2 -> CH 20 + 4S + 3H 2O.
How is hydrogen sulfide used in the chemosynthesis process?
Giant tube worms use bacteria in their trophosome to fix carbon dioxide (using hydrogen sulfide as an electron and oxygen or nitrate as an energy source) and produce sugars and amino acids. Some reactions produce sulfur:
Last Update: Oct 2021